Thermo Fisher Scientific › Electron Microscopy › Electron Microscopes › 3D Visualization, Analysis and EM Software › Use Case Gallery
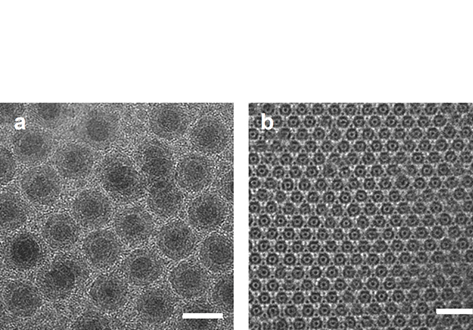
Core@shell Fe@Fe3O4 nanoparticles (NPs) are synthesized via the thermal decomposition of iron pentacarbonyl (Fe(CO)5) in the presence either of oleylamine (OAm) or a mixture of OAm and oleic acid (OA). The heterostructured nanocomposites formed do so by a postsynthetic modification of isolated Fe seeds. This proves the versatility of the coating procedure and represents a significant advantage over previous work with Co seeds owing to the higher magnetic susceptibility, reduced toxicity, and excellent biocompatibility of Fe. Furthermore, the latter system allows the synthetic methodology to be developed from a two‐pot scenario where seeds are isolated then coated, to an easier and more efficient direct one‐pot scenario. The two‐pot method yields proportionately larger cores. However, in both cases, the monodisperse product reveals a carbonaceous interface between the Fe core and oxide shell. Meanwhile for the one‐pot synthesis, the OA:OAm ratio influences both the morphology and dispersity of the product. This is interpreted in terms of competing interactions of the ligands with the iron precursor. Superparamagnetism (SPM) is observed, and microscopic studies reveal oxidative stability of the Fe(0) cores achieved by either method for >6 months. It is proposed that the carbonaceous interface is critical to this sustained oxidative stability.
For Research Use Only. Not for use in diagnostic procedures.