Thermo Fisher Scientific › Electron Microscopy › Electron Microscopes › 3D Visualization, Analysis and EM Software › Use Case Gallery
Li metal is considered as the most important negative electrode active material for Li-based batteries because of its high theoretical specific capacity of 3860mAh g-1, which is an order of magnitude higher than the currently used graphite, and by being the most electropositive metal. When coupled with high-capacity cathodes, either Li insertion materials or conversion chemistries, or applied in a solid-sate configuration, a leap in energy density can be obtained. The main challenge in the direct utilization of Li metal as anode is the formation of a mossy and dendritic morphology upon cycling manifested in a low Columbic efficiency and a continuous consumption of the electrolyte through parasitic side reactions, continuous growth of the solid electrolyte interphase (SEI) and irreversible capacity loss on the anode. These issues are a result of processes taking place both during charge (Li plating) and discharge (Li stripping), creating an unstable electrode structure.
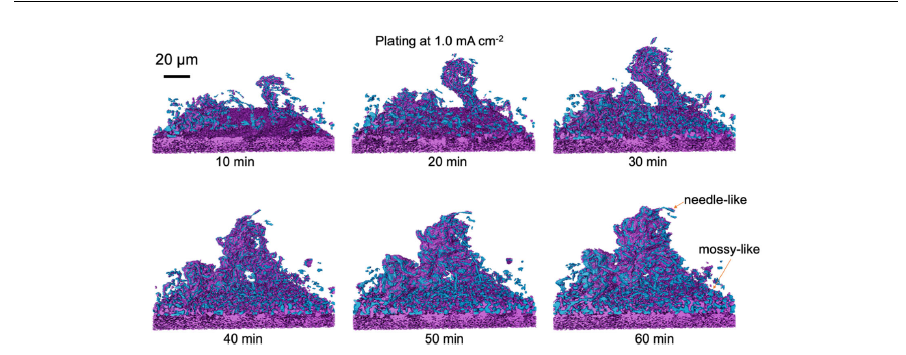
In this work, we demonstrate the use of operando synchrotron XTM to capture the formation, growth, and dissolution of Li microstructures.
The isolated regions of Li in Fig. 2 were determined by using the Label Analysis module of the AVIZO software package on the segmented Li volumes. […] The calculation of the object-specific surface of the Li microstructures in Fig. 2e is carried out using the Object Specific Surface module of AVIZO software […] 3D rendering of the segmented Li phase was performed using AVIZO, along with the volume analysis which calculated the volume by multiplying the number of segmented Li voxels, by the voxel volume (0.325 μm× 0.325 μm× 0.325 μm). The Li phase-specific surface area was then calculated by dividing the surface area of the Li phase by the Li volume. […]
For Research Use Only. Not for use in diagnostic procedures.