Thermo Fisher Scientific › Electron Microscopy › Electron Microscopes › 3D Visualization, Analysis and EM Software › Use Case Gallery
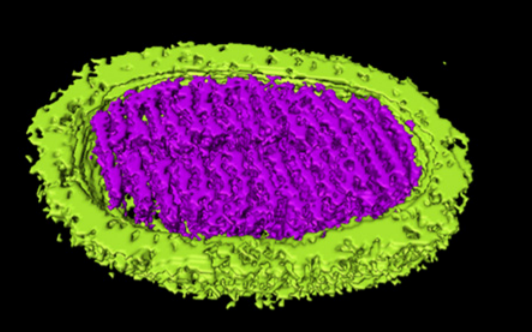
A novel archaeal virus, denoted Sulfolobus ellipsoid virus 1 (SEV1), was isolated from an acidic hot spring in Costa Rica. The morphologically unique virion of SEV1 contains a protein capsid with 16 regularly spaced striations and an 11-nm-thick envelope. The capsid exhibits an unusual architecture in which the viral DNA, probably in the form of a nucleoprotein filament, wraps around the longitudinal axis of the virion in a plane to form a multilayered disk-like structure with a central hole, and 16 of these structures are stacked to generate a spool-like capsid. SEV1 harbors a linear double-stranded DNA genome of ∼23 kb, which encodes 38 predicted open reading frames (ORFs). Among the few ORFs with a putative function is a gene encoding a protein-primed DNA polymerase. Sixfold symmetrical virus-associated pyramids (VAPs) appear on the surface of the SEV1-infected cells, which are ruptured to allow the formation of a hexagonal opening and subsequent release of the progeny virus particles. Notably, the SEV1 virions acquire the lipid membrane in the cytoplasm of the host cell. The lipid composition of the viral envelope correlates with that of the cell membrane. These results suggest the use of a unique mechanism by SEV1 in membrane biogenesis.
The ET volumes were segmented, surface rendered, and displayed in 3-D with Amira software . The volume segmentations were carried out with the Amira software. The ET volumes were segmented, surface rendered, and displayed in 3-D with Amira software.
For Research Use Only. Not for use in diagnostic procedures.