Thermo Fisher Scientific › Electron Microscopy › Electron Microscopes › 3D Visualization, Analysis and EM Software › Use Case Gallery
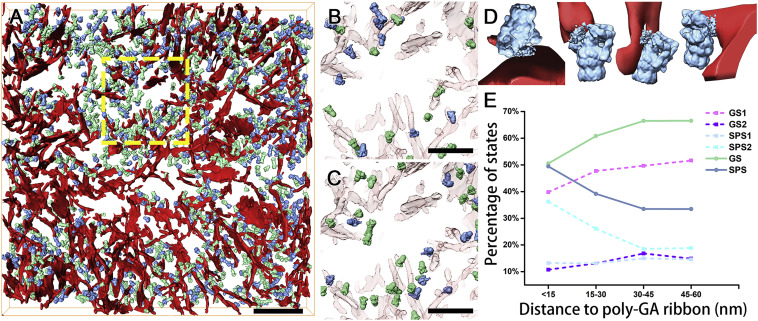
Protein aggregation and dysfunction of the ubiquitin-proteasome system are hallmarks of many neurodegenerative diseases. Here, we address the elusive link between these phenomena by employing cryo-electron tomography to dissect the molecular architecture of protein aggregates within intact neurons at high resolution. We focus on the poly-Gly-Ala (poly-GA) aggregates resulting from aberrant translation of an expanded GGGGCC repeat in C9orf72, the most common genetic cause of amyotrophic lateral sclerosis and frontotemporal dementia. (…) poly-GA aggregates may compromise neuronal proteostasis by driving the accumulation and functional impairment of a large fraction of cellular proteasomes.
Segmentations were visualized using Amira.
For Research Use Only. Not for use in diagnostic procedures.