Thermo Fisher Scientific › Electron Microscopy › Electron Microscopes › 3D Visualization, Analysis and EM Software › Use Case Gallery
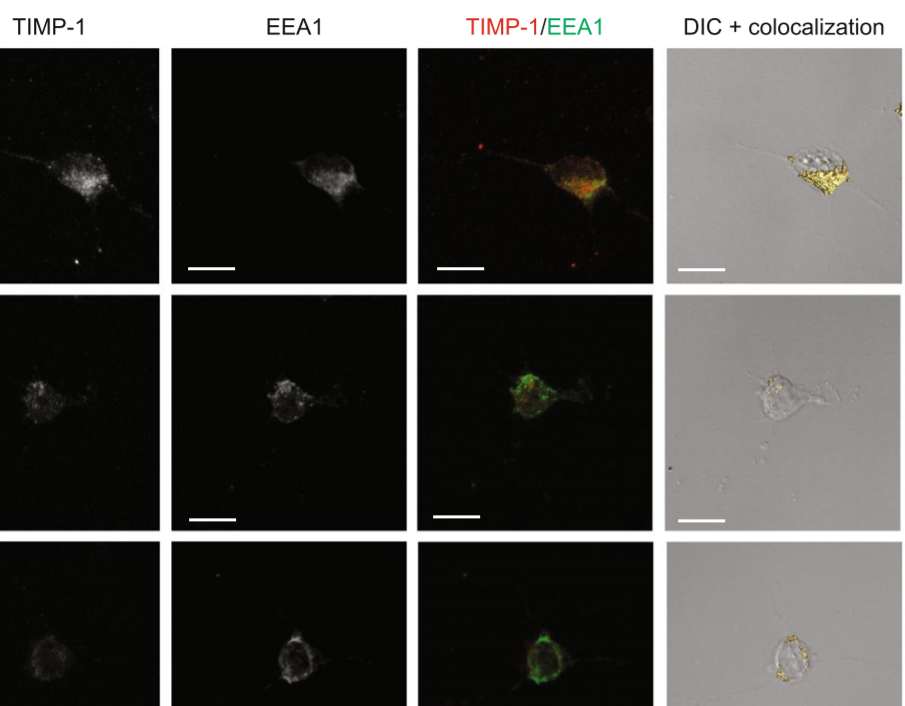
The tissue inhibitor of metalloproteinases-1 (TIMP-1) exerts inhibitory activity against matrix metalloproteinases and cytokine-like effects. We previously showed that TIMP-1 reduces neurite outgrowth in mouse cortical neurons and that this cytokine-like effect depends on TIMP-1 endocytosis mediated by the low-density lipoprotein receptor-related protein-1 (LRP-1). To gain insight into the interaction between TIMP-1 and LRP-1, we considered conformational changes that occur when a ligand binds to its receptor. TIMP-1 conformational changes have been studied using biomolecular simulations, and our results provide evidence for a hinge region that is critical for the protein movement between the N- and C-terminal TIMP-1 domains. In silico mutants have been proposed on residues F12 and K47, which are located in the hinge region. Biological analyses of these mutants show that F12A or K47A mutation does not alter MMP inhibitory activity but impairs the effect of TIMP-1 on neurite outgrowth. Interestingly, these mutants bind to LRP-1 but are not endocytosed. We conclude that the intrinsic dynamics of TIMP-1 are not involved in its binding to LRP-1 but rather in the initiation of endocytosis and associated biological effects.
Maximal intensity projection representations were realised using the AMIRA software program. Quantification of the TIMP-1/EEA1 colocalisation was performed using the AMIRA software program.
For Research Use Only. Not for use in diagnostic procedures.